MANAGEMENT SERVICES
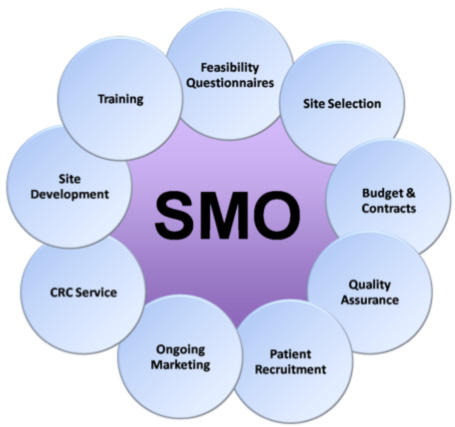
As a research and development services company, WMRAD delivers technical clinical trial management services to medical institutions and healthcare professionals. Our responsibilities include contract, Institutional Review Board submissions and notifications, patient recruitment, counseling and follow-up, informed consent procedures, site initiation and close-out operations, research-related documentation maintenance and archival, reporting adverse events to sponsors and regulatory authorities, ensuring protocol compliance, and advising investigators on potential protocol, and ICH-GCP violations.
- Contract and Budgeting
- Institutional Review Board submissions and notifications
- Patient Recruitment
- Patient Counseling and Follow-up
- Informed Consent Procedures
- Site Initiation and Close-out operations
- Research-related documentation maintenance and archival
- Reporting Adverse Events
- Ensuring protocol compliance
- Advising investigators on potential protocol, and ICH-GCP violation
CLINICAL TRIAL MANAGEMENT SOFTWARE
- Real-time metrics tracking
- Timeline reporting from study invitation to first patient consent forms
- Protocol Compliance
- Enrollment Quotas
- Case Report from Completion Metrics
- Deviations reports
- Interim Monitoring Visit Responses
- Integral Task lists
Become a Partner
WMRAD history is filled with stories of investigators, scientists and others who observed a problem and worked to solve it. The results of their accomplishments have been presented at professional meetings, documented in scientific journals and poster presentations and reported by the media.
WMRAD offers a comprehensive clinical research management program to assist sponsors with their clinical trial needs. Our research professionals assist sponsors and investigators to place, coordinate and/or facilitate clinical trials conducted at inpatient and outpatient facilities. The company understands sponsors needs and is committed to conducting clinical studies with heightened attention to time constraints and budgets.
If you would like to partner with us, fill out the form below and one of our staff members will get back to you.